Abstract
Introduction
Crizotinib, an inhibitor of ALK, MET and ROS1, is approved for patients with ALK-positive or ROS1-positive advanced non-small-cell lung cancer (NSCLC). However, ALK rearrangements are also implicated in other malignancies, including anaplastic large-cell lymphoma (ALCL) and inflammatory myofibroblastic tumors (IMT).
Methods
In this open-label, ongoing, multicenter, single-arm phase 1b study (PROFILE 1013 [NCT01121588]), male or female patients, aged ≥15 years, with ECOG performance status 0−3,with ALK-positive advanced malignancies other than NSCLC were to receive a starting dose of crizotinib 250 mg BID. Primary endpoints were safety and objective responses, based on RECIST v1.1 or NCI International Response Criteria.
Results
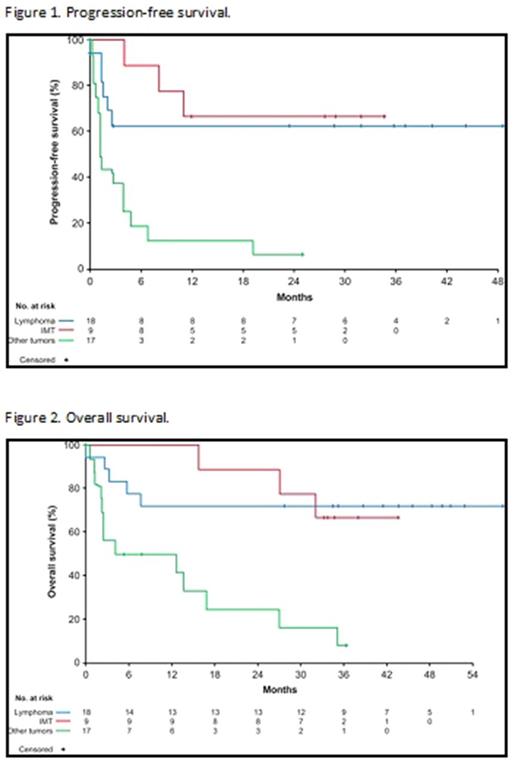
Forty-four patients were enrolled (lymphoma=18, IMT=9, other tumors=17). In the lymphoma group, 17/18 of patients were evaluable for response. Objective response rate was 52.9% (95% CI 27.8-77.0) for lymphoma, with 8 complete responses (CRs) and 1 partial response (PR); 66.7% (95% CI 29.9-92.5) for IMT, with 1 CR and 5 PRs; and 11.8% (95% CI 1.5-36.4) for other tumors, with 2 PRs in patients affected by colon carcinoma and medullary thyroid cancer, respectively.
Median treatment duration was close to 3 years for lymphoma patients (150.7 weeks [range 0.1-249.3]) and IMT patients (142.9 weeks [range 21.1-160.0]) and was 1 month for patients with other tumors. Treatment with any other anti-cancer treatment was not allowed.
In lymphoma patients, median PFS has not been reached due to a small number of events (33.3%) (Figure 1). The estimated probability of lymphoma patients being progression free at 2 years was 63.0% (95% CI: 35.3, 81.4). Median OS was also not reached as OS data were immature, with 72.2% of patients with lymphoma still alive at the cutoff (Figure 2). Only 1 patient received bone marrow transplant during crizotinib treatment and was censored for anti-tumor activity.
In IMT patients, median PFS was also not reached due to a small number of events (33.3%)(Figure 1). The estimated probability of IMT patients being progression free at 2 years was 66.7% (95% CI: 28.2, 87.8). In addition, median OS was not reached as 66.7% of patients with IMT were alive at data cutoff (Figure 2).
In the other tumors group median PFS was 1.3 months (95% CI: 1.1, 3.9) (Figure 1) with a high number of events (88.2%), PFS at 2 years was 6.3% (95% CI: 0.4, 24.7) and median OS was 8.3 months (95% CI: 2.2, 16.9) (Figure 2).
Treatment-related adverse events (TRAEs) occurred in a total of 39 (88.6%) patients. Mild to moderate diarrhea and vision disorder were the most common TRAEs reported (45.5%), most of which were grade 1. Overall, 9 patients (20.5%) experienced a serious adverse event (SAE) considered to be related to crizotinib treatment. The most common SAE considered as possibly treatment-related was grade 4 blood creatine phosphokinase increase reported in 3 patients (6.8%), all in clinical response and concurrent with strenuous physical activity. Grade 5 AEs considered by the investigator as treatment-related occurred in 2 patients: cerebral infarction in a patient with unknown primary tumor and multiple brain metastasis, concurrent with disease progression, and interstitial lung disease in a patient with nasopharyngeal carcinoma. Subsequent review by an independent radiology committee, consisting of a pulmonologist, radiologist and oncologist, indicated that this event was aspiration pneumonia, non-drug-related.
Conclusions
These findings indicate a strong and durable activity of crizotinib in ALK-positive lymphomas and IMTs. Treatment duration was much longer than usual treatment in NSCLC patients and the safety profile was consistent with the known safety profile of crizotinib, even with long-term treatment.
Gambacorti-Passerini: BMS: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding. Braiteh: Merck: Honoraria, Other: Travels, Speakers Bureau; Lexicon: Honoraria; Insys: Honoraria, Other: Travels, Speakers Bureau; Ipsen: Honoraria, Other: Travels, Speakers Bureau; Incyte: Honoraria, Other: Travels, Speakers Bureau; Heron Therapeutics: Honoraria, Other: Travels; Halozyme Therapeutics: Other: Travels; Eli Lilly: Honoraria, Other: Travels, Speakers Bureau; Clovis: Honoraria, Speakers Bureau; Celgene: Other: Travels, Speakers Bureau; Bristol Myers Squibb: Other: Travels, Speakers Bureau; Boehringer Ingelheim: Honoraria, Other: Travels, Speakers Bureau; Biotheranostics: Honoraria, Speakers Bureau; Bayer: Honoraria, Other: Travels; Astra Zeneca/Medimmune: Honoraria, Other: Travels, Speakers Bureau; Amgen: Honoraria, Other: Travels, Speakers Bureau; Merrimack: Honoraria, Other: Travels, Speakers Bureau; Pfizer: Honoraria, Other: Travels, Speakers Bureau; Roche/Genentech: Honoraria, Other: Travels, Speakers Bureau. Ahn: Boehringer Ingelheim: Honoraria; Menarini: Honoraria; Roche: Honoraria; Janssen: Honoraria; Eisai: Honoraria; BMS: Honoraria. Taylor: Eisai Inc.: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Trillium Therapeutics: Consultancy, Honoraria; Blue Print Medicines: Consultancy, Honoraria. Van Tine: Pfizer: Research Funding; Novartis: Consultancy, Speakers Bureau; Merch: Research Funding; Lilly: Consultancy, Speakers Bureau; J&J: Consultancy, Speakers Bureau; Caris: Consultancy, Speakers Bureau. Wu: Celgene International Sàrl: Research Funding. Paolini: Pfizer: Employment, Equity Ownership. Li: Pfizer: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal